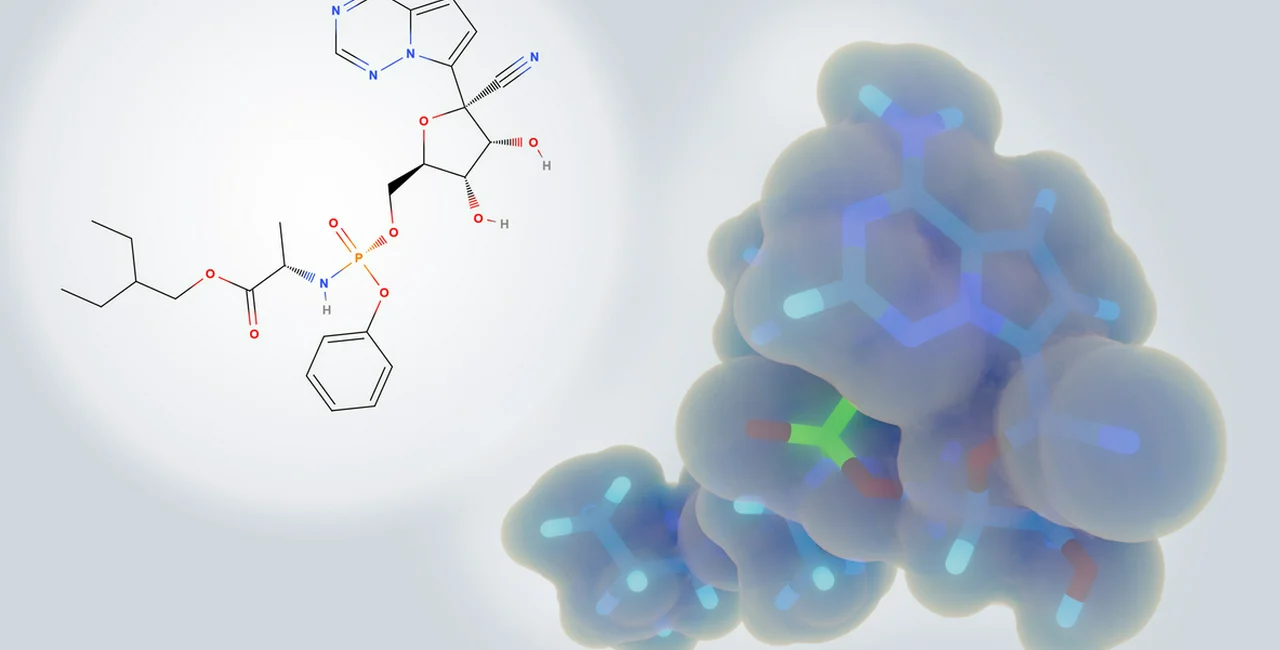
Prague, March 17 (CTK) – The Health Ministry has permitted the use of Remdesivir, an experimental medicine against COVID-19, in Czechia, according to the document it posted on its website today, after a Prague hospital demanded Remdesivir for a patient in a critical condition.
Remdesivir is an experimental medicine produced by the U.S. firm Gilead.
The Prague-based General Teaching Hospital (VFN) wants it for a patient who is in a critical condition with new coronavirus, VFN spokeswoman Marie Hermankova has told CTK.
The Czech Republic can address Gilead, apply for Remdesivir and describe the condition of the patient concerned, and Gilead would decide whether to make the patient a part of the relevant programme and provide the medicine for him.
Czech PM Andrej Babis (ANO) told journalists after the cabinet meeting earlier today that Prague would send a military plane to bring the medicine to Czechia.
The ministry’s permit of the Czech use of Remdesivir is temporary, with a six-month validity.
“The medicine is designated for the patients in the Czech Republic who have been diagnosed with the COVID-19 infection, are staying in hospital and their health condition requires their connection to a ventilator,” the ministry has written.
Hospitals would be bound to have the patients’ consent with being administered an experimental medicine that has not been officially approved yet.
The use of every new medicine in Czechia must be approved by the State Institute for Drug Control (SUKL), which assesses the medicines’ safety and effectiveness based on available information from the producer and also from the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
If the producer applies for it, the SUKL also assesses the possibility of the respective new medicine price’s coverage by public health insurers.
Remdesivir would probably be provided for free within the clinical research.
Among the current 434 coronavirus patients in Czechia, three are in a critical condition. One of them, a man, is in the VFN.
“The patient’s condition remains unchanged. He is staying at the clinic of anaesthesiology, resuscitation and intensive medicine, is in a serious condition, is connected to the Extracorporeal membrane oxygenation (ECMO), but is stable,” Hermankova said.
Jan Kvacek, director of Prague’s Bulovka hospital, told Czech Television tonight that he wants Remdesivir for a coronavirus patient in his hospital, who, too, is in a serious condition, connected to a ventilator.
The third patient in a critical condition, a woman connected to a ventilator, stays in Prague’s Military University Hospital (UVN).
There are currently 434 confirmed cases of coronavirus in the Czech Republic, and health officials have conducted 6,302 tests according to the Ministry of Health.
Three of the initial coronavirus cases recorded in the Czech Republic have recovered. The country has attributed no deaths to coronavirus.
This is a developing story. We will post more information as it becomes available.












 Reading time: 2 minutes
Reading time: 2 minutes