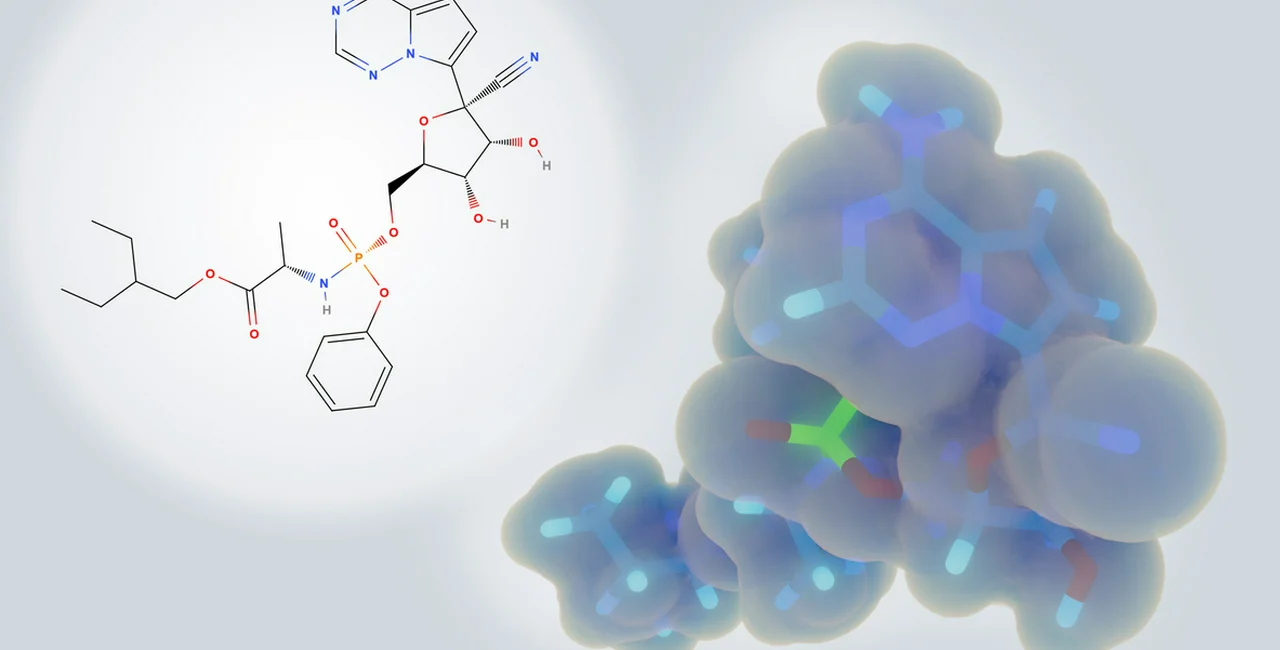
Prague, March 27 (CTK) – The condition of a Czech COVID-19 patient has markedly improved after the U.S. remdesivir experimental medicine was administered to him for three days, Marie Hermankova, the spokeswoman for Prague’s General Teaching Hospital (VFN), where the patient is treated, announced today.
Nevertheless, VFN director David Feltl told a press conference that the patient’s condition remains very serious and, in addition, it began improving still before remdesivir started to be given to him.
Feltl said the state mainly has to solve the problem with the testing capacities and the capacities of the medical staff who have been working on the brink of self-sacrifice.
The man, whom the VFN admitted with a lung failure earlier this month, may be disconnected from a cardiopulmonary bypass machine ECMO (extracorporeal membrane oygenation) that ensures his blood oxygenation, according to CTK’s information.
The man is Czechia’s only patient to whom the U.S. producer granted the remdesivir medicine. The application of another Prague hospital, Bulovka, has not been met.
Feltl said the VFN patient’s condition had been partly improving even before remdesivir being given to him.
“It [remdesivir] still amounts to experimental treatment. The situation is much more complicated, it cannot be simply said that a medicine has arrived and saved the patient,” Feltl said.
VFN Anaesthesiology Clinic deputy head Martin Balik said the therapy has been complex and that the VFN had waited a whole week for remdesivir’s arrival from the USA.
“This is a very long period of time, for which intensive care [doctors] cannot simply wait, they have to try to put the patient together,” he said.
The administration of remdesivir has not harmed the patient. “Nevertheless, we cannot be sure whether remdesivir is the key substance,” Balik emphasised.
Balik previously said the patient had his lungs damaged. First, he was admitted to Prague’s Thomayer hospital on March 10 with suspected lung inflammation. After five days, he was rushed to the VFN and connected to ECMO.
The Czech Republic has a total of 74 such apparatuses for the most severe COVID-19 cases. Further patients with severe symptoms mostly need ventilators and oxygen supplies.
The VFN gained the permit to apply remdesivir from Gilead, the U.S. company for which it has been developed by a team led by Czech Tomas Cihlar.
On Sunday, however, Gilead announced the suspension of its granting of licences for remdesivir experimental treatment, with the exception of patients such as pregnant women and underage children.
Czech Deputy Health Minister Roman Prymula said the Czech Republic is negotiating about the possibility to apply the medicine in a further phase of its development.
He said Europe might keep a stock with remdesivir for Gilead to approve its application to patients.
The first dose was supplied to Czechia for free, further ones would have to be paid for by the Czech state.
Remdesivir, originally developed to stem the Ebola spread in Congo, is an antiviral drug preventing the multiplication of virus. The World Health Organisation (WHO) has called it the most promising candidate for fighting the new coronavirus.












 Reading time: 2 minutes
Reading time: 2 minutes